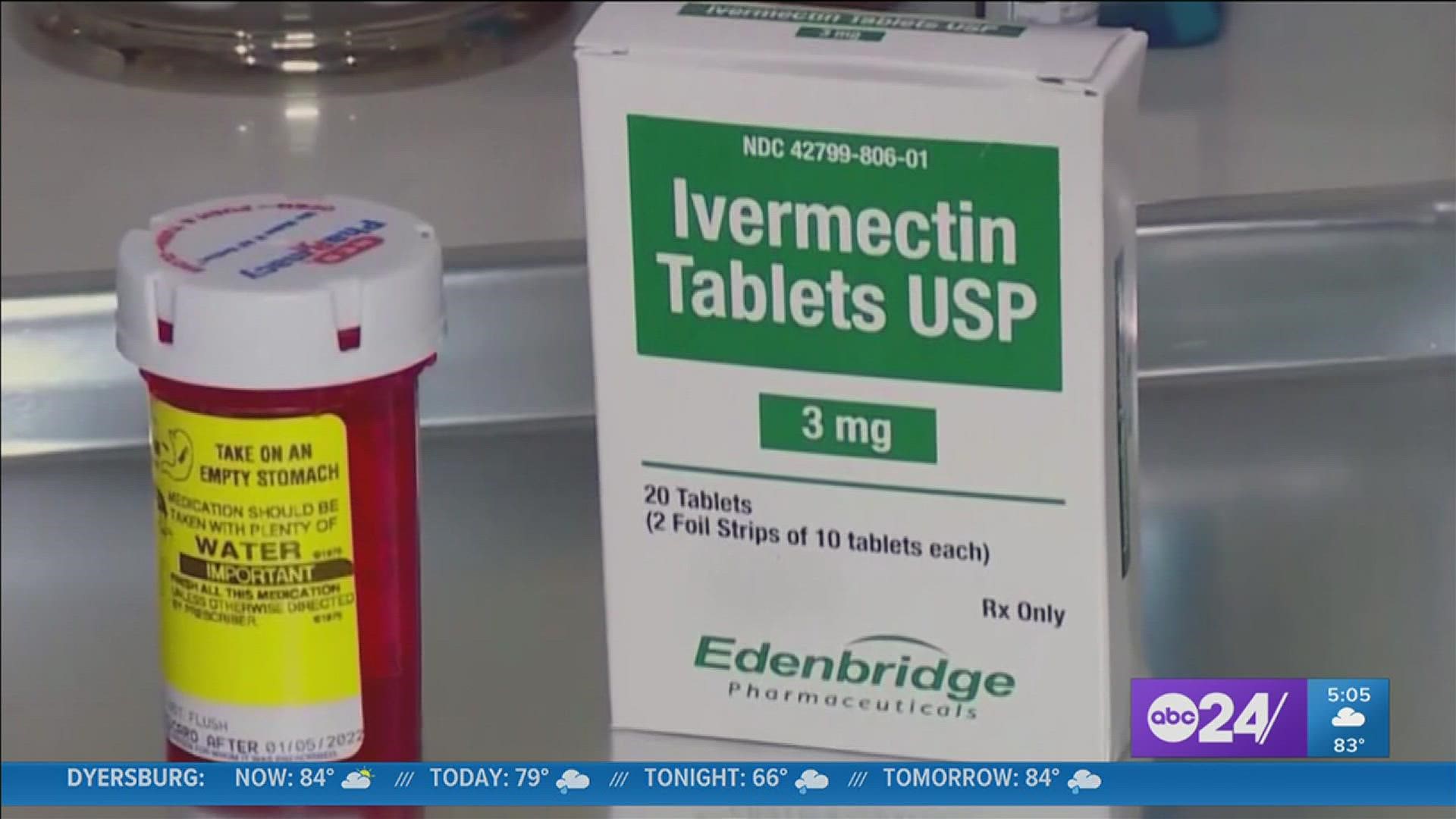
MEMPHIS, Tenn. — A national study is underway to see if Ivermectin and two other drugs already on the market are effective at treating COVID-19 symptoms.
The anti-parasite medicine has been popular among anti-vaccine advocates. Duke University researchers are now leading the nationwide trial and 15,000 participants are needed.
"Ivermectin has a lot of controversy. We’re hoping to clear that up," said Dr. Aaron Milstone with the Clinical Trials Center of Middle Tennessee.
Milstone said this trial should provide the medical community with the answers it needs when it comes to whether or not Ivermectin works as a COVID treatment.
"This is a much safer way to take ivermectin and it’s really going to clear the air for all of us," said Milstone. "The goal of the trial is to use medicines that are already FDA approved to see if these medicines might prevent someone with COVID from getting sicker and ending up in the hospital."
The study is called the ACTIV-6 trial. It is testing not only Ivermectin, but also Fluvoxamine, an anti-depressant, and Fluticasone, a cortico-steroid commonly used for asthma or COPD.
"This is a really important trial because we don’t have a lot of options for people who are not hospitalized," said Milstone.
Milstone works for Clinical Trials Center of Middle Tennessee. It and many major medical facilities across the country are helping to administer it.
To participate, you have to be 30 or older, have recently tested positive for COVID-19 and have not been in the hospital.
"If you live in Memphis, Union City or Jackson, you can participate in this trial without ever leaving your home. All of the medications in the study are dropped shipped to your home and there are two visits to the home but those visits are done remotely," said Milstone.
Milstone said participants are compensated for taking part in the study. He said if a person is in the study, they can also get Monoclonal antibody treatments, as well.
To participate, call 833-385-1880 or click here to sign up on the study's website.
For more information about the study, click here.