WASHINGTON — As cases of coronavirus spike across the country, the results of another vaccine trial bring cautious hope.
Drug company Moderna reports their vaccine has a 94.5% efficacy.
In the last few weeks we have had all sorts of information of vaccine trials coming in. But how did Moderna get to that magic number of 94.5%?
Question:
What is a vaccine’s efficacy? How did Moderna get their number?
Answer:
Our Sources:
The Centers for Disease Control and Prevention (CDC) and the results of the Moderna vaccine trials.
Our Process:
According to the CDC, the efficacy of a vaccine is a measure of the reduction of disease in a group that has been vaccinated in a study like a clinical trial.
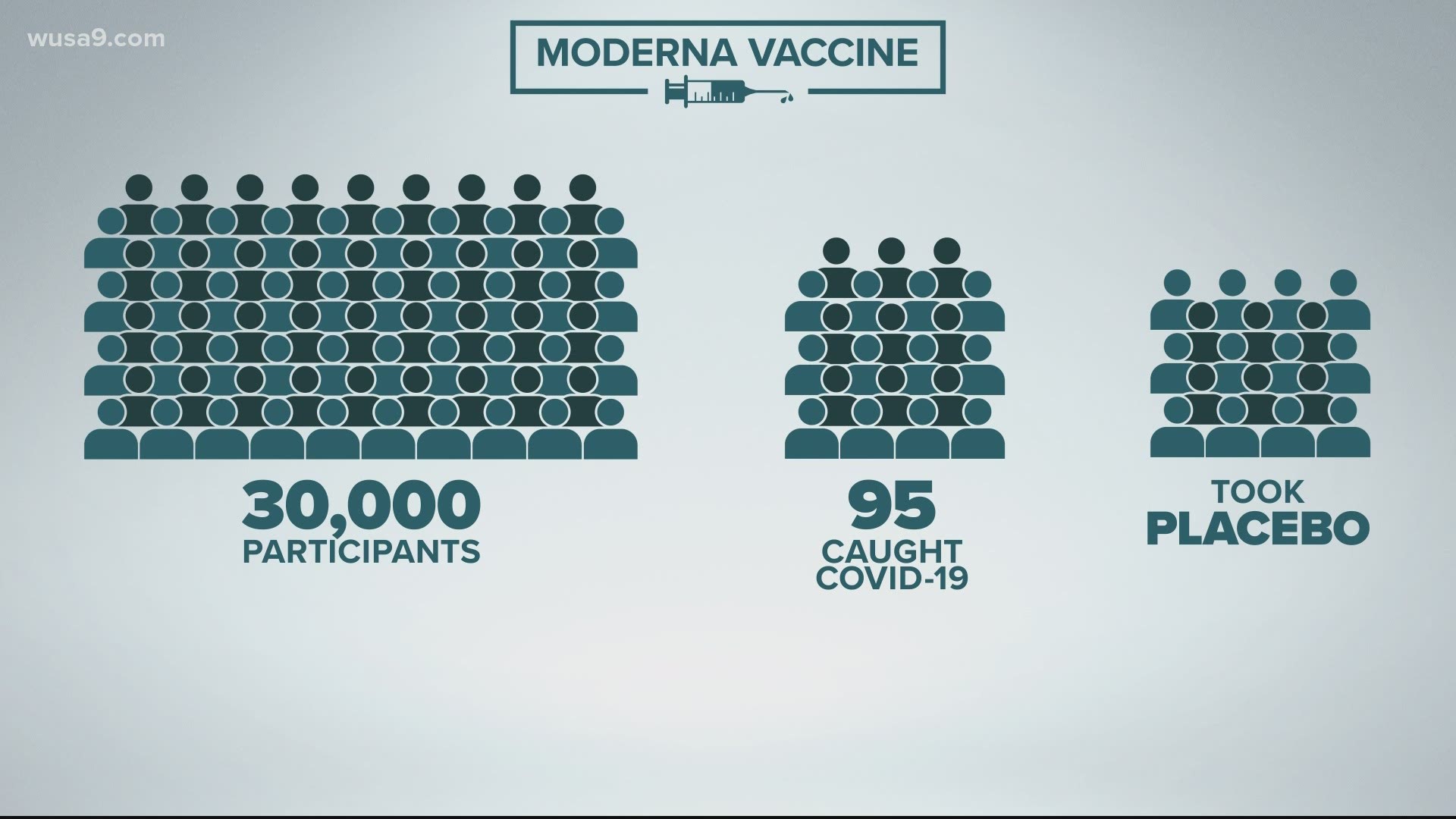
In Phase III of Moderna’s clinical vaccine trial, 30,000 people took part. Half got the vaccine, the other half received a placebo. Of the 30,000 people, 95 people got COVID-19.
From the 95 people who got the virus, 90 received the placebo. The other five received the vaccine. Moderna’s math showed that after two months the vaccine had a 94.5% efficacious rate.
So far this fits the Food and Drug Administration’s emergency standard. In June, the FDA wanted a vaccine to reduce or prevent COVID in 50% or more people who got the vaccination.
But it’s important to note, the Moderna trial shows efficacy in a controlled-small group. There is no guarantee that everyone in the study was exposed to the virus.
A vaccine’s real test of effectiveness, in the long run, is tested in the general population